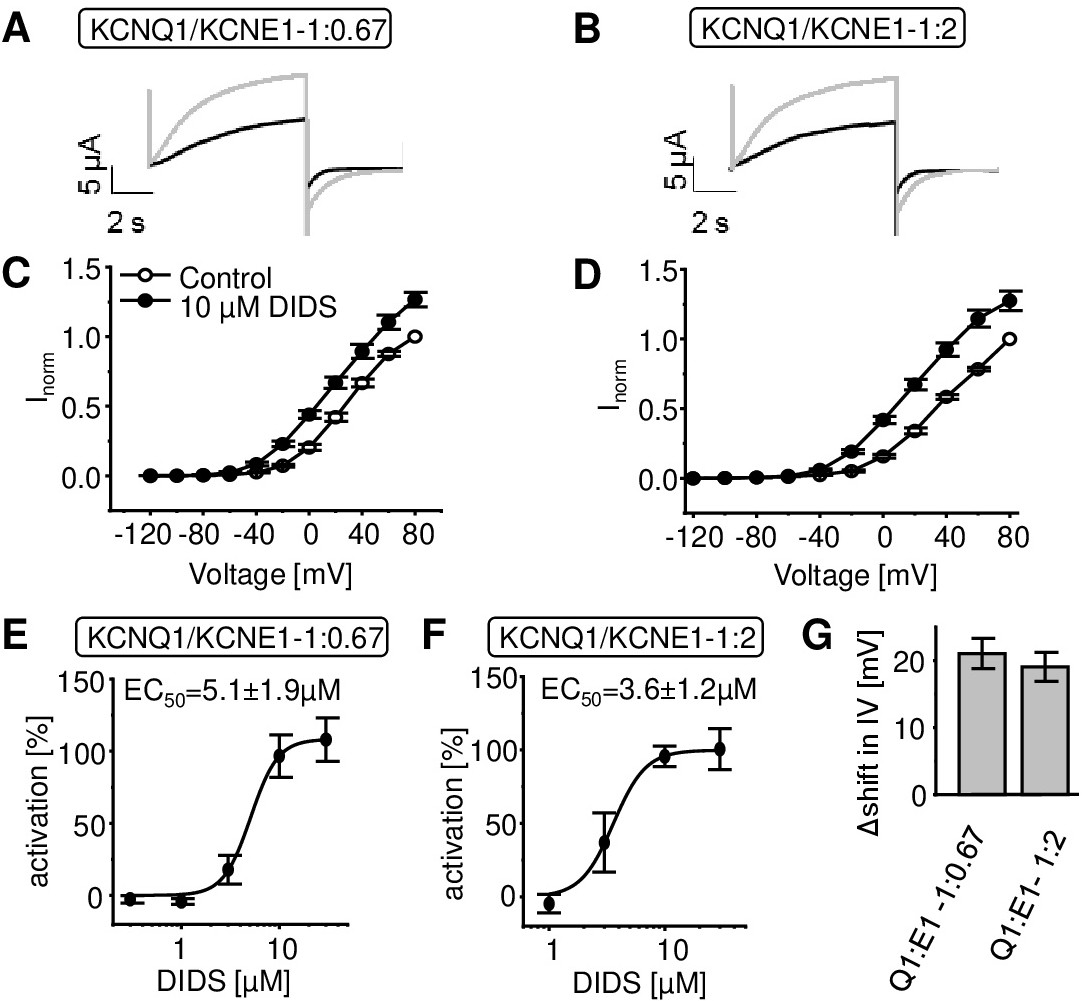
Fig. 2. Impact of varying KCNQ1/KCNE1 stoichiometry on concentration dependence of DIDS action and change in current/voltage relationship. A,B. Representative current traces of KCNQ1-WT/KCNE1-WT at two KCNQ1/KCNE1 ratios before and after application of 10 ÁM DIDS. C,D. Tail currents were analyzed at -120 mV and normalized to tail currents at +80 mV before application of 10 ÁM DIDS (Q1/E1 1:0.67 n = 7; Q1/E1 1:2 n = 6 ▒ SEM). E,F. Dose-response relationship for activation of KCNQ1/KCNE1 channel currents at two KCNQ1/KCNE1 ratios. Activation was determined as percent change in current amplitude at +40 mV. Current amplitudes were measured before and after a series of 15 voltage equilibration pulses. Data were fitted to a logistical function to calculate EC50-value. (Q1/E1 1:0.67 n = 3-21; Q1/E1 1:2 n = 3-38; ▒ SEM). G. V1/2-values were calculated by Boltzmann-fits of each individual oocyte's normalized peak tail currents before and after application of 10 ÁM DIDS (n = 6-14, ▒ SEM). Even though the currents did not saturate V1/2 values can be approximated. DIDS causes a shift in voltage-dependence of KCNQ1/KCNE1 channel activation (Q1/E1 1: 0.67 - V1/2 = 33.5 ▒ 2.1 mV shifted to V1/2 = 12.4 ▒ 2.2 mV (n = 14), Q1/E1 1: 2 V1/2 = 40.2 ▒ 1.9 mV shifted to V1/2 = 21.2 ▒ 2.6 mV (n = 6)). Shift in half maximal activation voltage V1/2 is not significantly influenced by varying the KCNQ1/KCNE1 ratios. Data were tested for significance by performing two sample t-test.